- Service Details
- Demo
- Case Study
- Publications
What are Amino Sugars?
Amino sugars are chemical compounds that have a sugar backbone, in which one of the hydroxyl groups is replaced by an amine group. Derivatives of amine-containing sugars, such as N-acetylglucosamine, are also considered amino sugars. Incorporated into protein-linked sugar chains, amino sugars regulate protein function and, combined with other compounds, form antibiotics.
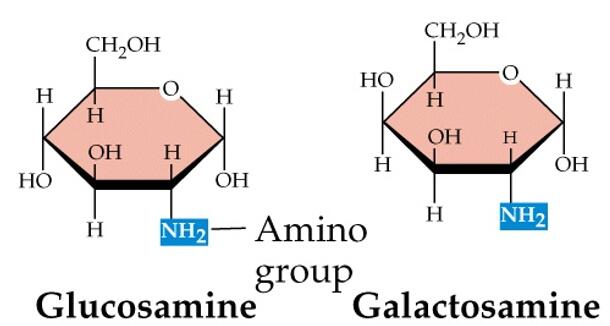 Figure. Amino sugars. The monosaccharides glucosamine and galactosamine are amino sugars with the amino group in place of a hydroxyl group.
Figure. Amino sugars. The monosaccharides glucosamine and galactosamine are amino sugars with the amino group in place of a hydroxyl group.
Amino sugars are widely associated with structural polysaccharides or glycolipids, and they have been proposed as biomarkers for organic matter sources and their digenetic state. Three vital amino sugars are of physiological importance, namely glucosamine, galactosamine and neuraminic acid. Glucosamine and galactosamine are derived from glucose and galactose respectively, the amino group in each case replacing the hydroxyl group on C-2. Neuraminic acid is more complex. It is a nine-carbon amino sugar formed by the condensation of mannosamine 6-phosphate and phosphoenolpyruvate (PEP). It is the parent substance of the sialic acids that are acyl derivatives of neuraminic acid, e.g. N-acetylneuraminic acid. Sialic acids are found as constituents of glycoproteins and glycolipids and are believed to have an important bearing on the formation of the acquired integuments of the teeth.
 Figure 2. The important function of amino sugars.
Figure 2. The important function of amino sugars.
Creative Proteomics offers metabolomics services for the comprehensive analysis of amino sugars. Our expertise in metabolomics enables precise identification and quantification of these compounds in various sample types, catering to both research and industrial needs.
Amino Sugars Analysis in Creative Proteomics
Glucosamine Analysis: Accurate quantification and structural characterization of glucosamine using high-performance liquid chromatography (HPLC) and mass spectrometry (MS) technologies.
Galactosamine Analysis: Determination of galactosamine presence and concentration in samples using advanced separation and detection techniques.
N-Acetylglucosamine Analysis: Analysis of N-acetylglucosamine and derivatives using specialized HPLC methods, supporting research in carbohydrate and lipid metabolism.
Sialic Acid Analysis: Quantification and structural analysis of Sialic Acid using liquid chromatography and mass spectrometry, applicable to biological samples and drug development.
Mannosamine Analysis: Precision analysis of mannosamine, supporting quantitative studies of amino sugars in biological samples.
Muramic Acid Analysis: Distribution and quantification of muramic acid in microbial and environmental samples using highly sensitive HPLC methods.
Analytical Techniques for Amino Sugars Analysis
High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography is a cornerstone technique utilized for separating and quantifying amino sugars in complex samples. At Creative Proteomics, we utilize:
- Agilent 1290 Infinity II LC System: Known for its robustness and high-resolution capabilities, ideal for separating amino sugars with varying structural complexities.
- Thermo Scientific Dionex Ultimate 3000 UHPLC System: Equipped with advanced detectors and automation features, ensuring rapid and accurate analysis of amino sugars.
Coupling HPLC with Mass Spectrometry enhances the specificity and sensitivity of amino sugar analysis. Key instruments include:
- Thermo Scientific Q Exactive HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer: Offering high-resolution accurate mass (HRAM) capabilities for precise identification and quantification of amino sugars.
- AB SCIEX Triple Quad 6500+ LC-MS/MS System: Known for its robustness in targeted quantitation, crucial for complex biological matrices containing amino sugars.
For specific applications, Gas Chromatography coupled with Mass Spectrometry is employed, particularly for volatile derivatives of amino sugars. Instruments include:
- Agilent 7890B GC System with 5977A Mass Selective Detector: Ensures sensitive detection of amino sugar derivatives after derivatization, crucial for structural elucidation and trace-level analysis.
Amino Sugars and Related Acids Analyzed at Creative Proteomics
| List of Amino Sugars and Related Acids Analyzed | |
|---|---|
| Amino Sugars | Related Acids |
| Glucosamine | N-Acetylglucosamine |
| Galactosamine | Muramic acid |
| N-Acetylglucosamine | N-Acetylgalactosamine |
| Mannosamine | N-Acetylneuraminic acid |
| Sialic acid | L-Daunosamine |
| Muramic acid | Neuraminic acid |
| N-Acetylgalactosamine | N-Acetylmannosamine |
| N-Acetylneuraminic acid | Galacturonic acid |
| L-Daunosamine | Mannuronic acid |
| Neuraminic acid | Glucuronic acid |
| N-Acetylmannosamine | Galacturonic acid |
| Galacturonic acid | Glucuronic acid |
| Mannuronic acid | |
Sample Requirements for Amino Sugars Analysis
| Sample Type | Recommended Amount | Notes |
|---|---|---|
| Biological Tissues Examples: liver, muscle, brain tissue. | 10-50 mg | Homogenize thoroughly to ensure representative sampling; store at -80°C. |
| Cell Cultures Examples: cell lines, primary cells. | 1-5 million cells | Maintain in appropriate growth media; collect at optimal confluence. |
| Body Fluids Examples: serum, plasma, urine, cerebrospinal fluid. | 0.5-1 mL | Collect using appropriate tubes; centrifuge immediately; store at -20°C. |
| Microbial Samples Examples: bacterial cultures, environmental samples. | Varies | Collect under sterile conditions; store frozen or in appropriate growth media. |
| Food and Feed Samples Examples: grains, animal feed, processed foods. | 1-5 grams | Grind to fine powder if solid; ensure representative sampling for homogeneity. |
Important Considerations:
- Sample Handling: Ensure sterile techniques for microbial samples; avoid repeated freeze-thaw cycles.
- Sample Preparation: Tailor extraction methods to sample type (e.g., tissue homogenization, cell lysis, protein precipitation).
- Storage Conditions: Maintain samples at recommended temperatures to preserve metabolite stability (e.g., -80°C for tissues, -20°C for fluids).
Report
- A detailed technical report will be provided at the end of the whole project, including the experiment procedure, instrument parameters.
- Analytes are reported as uM or ug/mg (tissue), and CV's are generally<10%.
- The name of the analytes, abbreviation, formula, molecular weight and CAS# would also be included in the report.

PCA chart

PLS-DA point cloud diagram

Plot of multiplicative change volcanoes

Metabolite variation box plot

Pearson correlation heat map
Reevaluation and novel insights into amino sugar and neutral sugar necromass biomarkers in archaea, bacteria, fungi, and plants.
Journal: Science of the Total Environment
Published: 2024
Background
Soil organic matter (SOM) constitutes the largest reservoir of terrestrial organic carbon (C), primarily influenced by soil microbes, which play a crucial role in its formation and long-term storage. Microbial necromass, comprised mainly of dead microbial biomass, such as cell wall residues and extracellular polymeric substances (EPS), constitutes a significant portion of SOC. This necromass is stabilized through organo-mineral interactions, organic-organic associations, and necromass-necromass interactions. Amino sugars like Glucosamine (GlcN) and Muramic acid (MurA) are prominent biomarkers used to trace microbial necromass in soils due to their specific origins in fungal chitin and bacterial peptidoglycan, respectively. Neutral sugars also serve as potential biomarkers, distinguishing between microbial and plant-derived polysaccharides in soil ecosystems. Despite advancements in biomarker research, there remain gaps in understanding the variability of these biomarkers among different microbial groups and plants, highlighting the need for further investigation and development of new biomarkers and conversion factors.
Materials & Methods
Standards and Reagents:
Amino sugar and neutral sugar standards (e.g., GlcN, MurA, GalN, Glc, Ara, Xyl) sourced from Sigma-Aldrich were employed. Chemical derivatization utilized 1-phenyl-3-methyl-5-pyrazolone (PMP) to facilitate analysis.
Sample Biomass Preparation:
Biomass from 120 species representing archaea (13), bacteria (23), fungi (56), and plants (28) was analyzed. Microbial biomass underwent freeze-drying, while plant materials were dried at 70°C. Acid hydrolysis with 6 M HCl and subsequent PMP derivatization were conducted as described.
UPLC-Orbitrap MS System:
Analysis was performed using a UPLC Ultimate 3000 system coupled to an Orbitrap Q Exactive HRMS with H-ESI source. Separation was achieved using a Waters AccQ. Tag Ultra C18 column, with data acquisition and processing following established protocols.
Targeted mass-to-charge ratios (m/z) were used for the identification and quantification of amino sugars and neutral sugars. Data analysis utilized FreeStyle™ 1.7 software for spectral processing and compound quantification.
Statistical comparisons among taxonomic groups were conducted using Kruskal-Wallis, Dunn, and Wilcoxon tests to assess differences in sugar content. Spearman correlations examined relationships such as between MurA and GlcN in bacterial groups.
Non-metric multidimensional scaling (NMDS) was employed to visualize clustering patterns based on sugar profiles. Analysis of similarity (ANOSIM) tested for significant group distinctions. Partial least squares discriminant analysis (PLS-DA) identified discriminatory metabolites among taxa.
Indicator Species Analysis:
Biomarkers associated with specific taxa were identified using the multipatt function from the indicspecies package, with multiple permutations ensuring robustness.
Results
Amino Sugar and Neutral Sugar Composition Across Taxa
We conducted comprehensive analyses of amino sugar and neutral sugar contents in biomass samples from archaea, bacteria, fungi, and plants using advanced metabolomic techniques.
Amino Sugar Profiles:
- Glucosamine (GlcN): Predominant in all groups, highest in fungi (45.9 μg/mg dw) and lowest in plants (1.4 μg/mg dw).
- Muramic Acid (MurA): Exclusive to bacteria, significantly higher in gram-positive (24.7 μg/mg dw) than gram-negative bacteria (3.9 μg/mg dw). Positive correlation with GlcN observed in gram-positive bacteria.
- Galactosamine (GalN): Highest in archaea (15.2 μg/mg dw), lower in bacteria, fungi, and plants (1.1-4.0 μg/mg dw).
- Talosaminuronic Acid (TalA): Found only in Euryarchaeota (6.4 μg/mg dw).
Neutral Sugar Profiles:
- Hexoses (Glucose, Galactose, Mannose): Predominant neutral sugars, with higher content in fungi and plants compared to bacteria and archaea.
Biomarker Significance and Taxonomic Differentiation
NMDS Ordination:
Amino sugar biomarkers distinguish gram-positive and gram-negative bacteria from archaea, fungi, and plants. MurA content is pivotal for bacterial separation.
 Fig. 2. Non-metric multidimensional scaling (NMDS) analysis based on amino sugar contents. Ellipses represent 95% confidence intervals.
Fig. 2. Non-metric multidimensional scaling (NMDS) analysis based on amino sugar contents. Ellipses represent 95% confidence intervals.
PLS-DA Discrimination:
Discriminant analysis reveals distinct metabolic profiles among bacteria, fungi, and plants based on amino and neutral sugar contents. GlcN, MurA, Glc, and Gal are key discriminatory markers.
 Fig. 3. Partial least squares discriminant analysis (PLS-DA) score plot based on amino sugar and neutral sugar contents.
Fig. 3. Partial least squares discriminant analysis (PLS-DA) score plot based on amino sugar and neutral sugar contents.
Indicator Species Analysis:
Specific biomarkers like GlcN for archaea, bacteria, and fungi, MurA for bacteria, and Glc and Gal for fungi and plants indicate taxon associations.
Implications for Ecological Roles and Conversion Factors
Conversion Factors:
Estimated factors convert sugar biomarkers into microbial necromass C, highlighting significant variations across taxa.
These findings underscore the metabolic diversity and ecological implications of sugar biomarkers in delineating taxonomic distinctions and ecological roles within soil microbial communities and plant interactions.
Reference
- Salas, Erika, et al. "Reevaluation and novel insights into amino sugar and neutral sugar necromass biomarkers in archaea, bacteria, fungi, and plants." Science of the Total Environment 906 (2024): 167463.
Combined omics approaches reveal distinct mechanisms of resistance and/or susceptibility in sugar beet double haploid genotypes at early stages of beet curly top virus infection.
Galewski, P. J., Majumdar, R., Lebar, M. D., Strausbaugh, C. A., & Eujayl, I. A.
Journal: International Journal of Molecular Sciences
Year: 2023
https://doi.org/10.3390/ijms241915013
Cancer SLC43A2 alters T cell methionine metabolism and histone methylation.
Bian, Y., Li, W., Kremer, D. M., Sajjakulnukit, P., Li, S., Crespo, J., ... & Zou, W.
Journal: Nature
Year:2020
https://doi.org/10.1038/s41586-020-2682-1
Metabolomic profiling implicates mitochondrial and immune dysfunction in disease syndromes of the critically endangered black rhinoceros (Diceros bicornis)
Corder, M. L., Petricoin, E. F., Li, Y., Cleland, T. P., DeCandia, A. L., Alonso Aguirre, A., & Pukazhenthi, B. S.
Journal: Scientific Reports
Year: 2023
https://doi.org/10.1038/s41598-023-41508-4
Transcriptomics, metabolomics and lipidomics of chronically injured alveolar epithelial cells reveals similar features of IPF lung epithelium
Willy Roque, Karina Cuevas-Mora, Dominic Sales, Wei Vivian Li, Ivan O. Rosas, Freddy Romero
Journal: bioRxiv
Year: 2020
https://doi.org/10.1101/2020.05.08.084459







