Title: Pharmacokinetic Comparisons of Naringenin and Naringenin-Nicotinamide Cocrystal in Rats by LC-MS/MS
Journal: BioMed Research International
Published: 2020
Background
Naringenin (NAR) is a flavonoid with various pharmacological activities, including antioxidation, anti-tumor, anti-inflammatory, and immunoregulatory effects. However, it suffers from poor water solubility and low bioavailability. To address these issues, the study investigates the pharmacokinetics and bioavailability of a naringenin-nicotinamide cocrystal (NAR-NCT), which is designed to enhance the solubility of NAR. The study evaluates the relative bioavailability, absorption rates, and elimination patterns of NAR and NAR-NCT in rats, demonstrating that NAR-NCT offers improved bioavailability, faster absorption, and slower elimination compared to NAR.
Materials & Methods
Materials
- Naringenin (NAR) (>96%) and nicotinamide (NCT) (>98%) were obtained from Bailingwei Technology (Beijing, China).
- The internal standard (IS), hesperetin (>98%), was from YIFEI (Shanghai, China).
- Acetic acid was from TEDIA (Fairfield, OH, USA).
- Methanol (HPLC grade), ethyl acetate (GC grade), and other chemicals were supplied by ANPEL (Shanghai, China).
Preparation and Characterization of NAR-NCT
- The NAR-NCT cocrystal was prepared via solvent evaporation. NAR (50 mg) and NCT (45 mg) were mixed in ethyl acetate (10 mL) at a molar ratio of 1:2. After ultrasonic dissolution, the mixture was heated at 40°C for 4 hours.
- Differential scanning calorimetry (DSC) and X-ray powder diffraction (XRPD) were performed to analyze NAR-NCT.
- Infrared spectroscopy (IR) was conducted using a Spectrometer 400 Fourier-infrared spectrometer (PerkinElmer, Waltham, USA).
- NAR-NCT stability was evaluated at 60°C, 90 ± 5% relative humidity, and 5000 Lx light for 10 days and after 6 months at room temperature.
Instrumentation
- Chromatographic separation was achieved with a Shimadzu UFLC-20AD XR system (Shimadzu, Tokyo, Japan) using a Shim-Pack C18-ODS column (75 mm × 3 mm, 2.3 μm) at 40°C and a flow rate of 0.3 mL/min.
- The mobile phase consisted of 0.2% acetic acid-water (v/v, A) and methanol (B).
- Mass spectrometry was performed using an AB SCIEX Qtrap 5500 (MDS-Sciex, Concord, Canada) with negative-mode electrospray ionization (ESI) interface.
Standard Working Solutions and Internal Standard Solution
- NAR was dissolved in methanol to make a 102.6 μg/mL stock solution, which was diluted to prepare standard working solutions.
- Hesperidin (300 ng/mL) was used as the internal standard solution.
Calibration Standards and Quality Control (QC) Samples
- Calibration standards were prepared in blank plasma at concentrations ranging from 1.03 to 821 ng/mL.
- Quality control samples were formulated at low, medium, and high concentrations corresponding to NAR of 3.08, 410, and 616 ng/mL, respectively.
Pretreatment of Plasma Samples
- Plasma (100 μL) was combined with 10 μL of the internal standard solution (300 ng/mL), vortexed for 5 minutes, and then mixed with ethyl acetate (300 μL).
- After vortex mixing for 5 minutes, the mixture was centrifuged at 8000 r/min for 2 minutes. Supernatants were collected, dried under nitrogen, and reconstituted with methanol (100 μL) for analysis.
Method Validations
- Specificity: Blank plasma and plasma samples were tested for interference by endogenous substances and metabolites.
- Calibration Curve and Lower Limit of Quantification (LLOQ): The calibration curve was established by analyzing plasma samples spiked with known concentrations of NAR. The LLOQ was determined by assessing reliability of quantification at the lowest concentration.
- Precision and Accuracy: Precision and accuracy were evaluated using standard plasma samples at various concentrations (LLOQ, LQC, MQC, HQC), with intra- and interday variations calculated.
- Extraction Recovery: Recovery was assessed by comparing the peak area of NAR in treated plasma to the peak area of untreated plasma.
- Matrix Effect (ME): ME was calculated by comparing the ratio of NAR peak areas in the plasma matrix to that in standard solutions.
- Stability: Stability studies were performed under various conditions (room temperature, freeze-thaw cycles, and long-term storage).
- Dilution Reliability: Dilution reliability was assessed for plasma samples with concentrations exceeding the calibration curve range.
Pharmacokinetic Study
- Eighteen SD rats (200 ± 20 g, 8 weeks old) were used. The rats were fasted for 12 hours before dosing, and they were divided into three groups for oral administration of NAR, NAR + NCT (1:2 molar ratio), and NAR-NCT (equivalent NAR dose of 30 mg/kg).
- Blood samples (0.5 mL) were collected at various time points (0.05 to 24 hours post-administration) and centrifuged for plasma separation, which was stored at −80°C.
Data Analysis
- Pharmacokinetic parameters were calculated using DAS Software (version 3.2.4) through noncompartmental modeling.
- Relative bioavailability (Fr) was calculated using the formula:
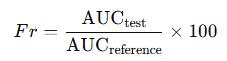
where AUC represents the area under the plasma concentration-time curve for each formulation.
Results
NAR-NCT Cocrystal
The NAR-NCT cocrystal was successfully prepared and characterized, showing improved water solubility. The differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), and infrared (IR) spectra confirmed the formation of a new NAR-NCT cocrystal, distinct from previously reported naringenin-nicotinamide cocrystals.
Stability of NAR-NCT
The stability of NAR-NCT was tested under various conditions, including high temperature (60°C), high humidity (90% RH), and light exposure. No significant changes were observed in the appearance or XRPD spectra of NAR-NCT, indicating that the cocrystal remained stable after 10 days under these conditions and after 6 months at room temperature.
 The spectrum of NAR-NCT
The spectrum of NAR-NCT
Optimization of Plasma Sample Preparation
Different extraction methods were evaluated for sample preparation. Ethyl acetate was found to be the most effective solvent for extraction, with a volume of 300 μL providing the best results.
Method Validation
The method for analyzing NAR in plasma using LC-MS/MS was validated. The calibration curve showed good linearity (r = 0.9995) and precision. The recovery rate for NAR ranged from 69.0% to 76.5%, with matrix effects between 107.7% and 131.2%. The plasma samples demonstrated good stability across different conditions (e.g., short-term, freeze-thaw cycles, long-term storage), meeting validation criteria.
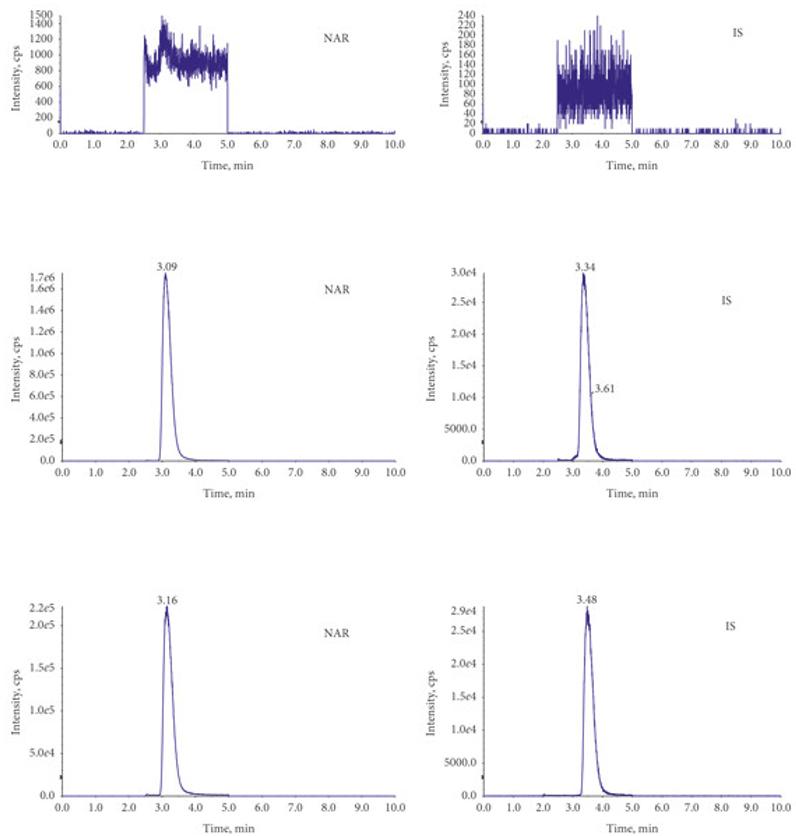 The chromatograms of MRM
The chromatograms of MRM
Pharmacokinetics and Bioavailability
Pharmacokinetic studies showed significant improvements in NAR bioavailability when administered as NAR-NCT. The maximum plasma concentration (Cmax) of NAR-NCT was 8.43 times higher than that of NAR, and the time to reach maximum concentration (Tmax) was reduced from 0.49 hours (NAR) to 0.09 hours (NAR-NCT). The relative bioavailability of NAR-NCT was 175.09% compared to NAR. NAR-NCT also showed slower elimination (longer half-life, t1/2 = 8.24 hours) and increased area under the curve (AUC), demonstrating faster absorption and sustained release.
Drug-Time Curve
The pharmacokinetic data for NAR, NAR + NCT, and NAR-NCT showed distinct differences. The drug-time curve for NAR-NCT exhibited a double peak, suggesting enterohepatic circulation, which extended the drug's action time in the body. The Cmax and Tmax for NAR-NCT were significantly improved compared to NAR, supporting the hypothesis that NAR-NCT enhances absorption and prolongs action.
Reference
- Xu, Dan, et al. "Pharmacokinetic comparisons of naringenin and naringenin‐nicotinamide cocrystal in rats by LC‐MS/MS." Journal of analytical methods in chemistry 2020.1 (2020): 8364218. https://doi.org/10.1155/2020/8364218






