- Service Details
- Demo
- Case Study
- FAQ
- Publications
As a family of enzyme cofactors, folate is a B-vitamin carries and chemically activates one-carbon units for biosynthesis of formate, formaldehyde, and methanol. One-carbon metabolism is a metabolic network widely existing in the cytoplasm, mitochondria, and nucleus. They are the interdependent and indespensible biosynthetic pathways in these organs. One-carbon metabolism in the cytoplasm is required for polyamine synthesis and methylation reactions of a wide range of molecules such as phospholipids, neurotransmitters, proteins and numerous other small molecules. In the mitochondria, the one-carbon metabolism is also needed for a large number of reactions, such as the interconversion of serine and glycine, the metabolism of choline, purines, and histidine and the synthesis of formylated methionyl-tRNA. Besides, the one-carbon metabolism in the mitochondria provides most of the one-carbon units used for metabolism in cytoplasm. In the nucleus of certain cell types, the folate-dependent de novo thymidylate biosynthesis pathway is shown to exist, which is indicated by increasing evidence.
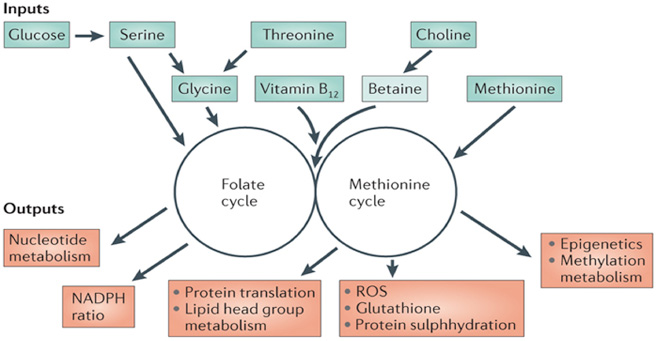
Function Of One-carbon Metabolism
Because of their relations to numerous diseases and abnormal physiological conditions range from birth to senescence, there is an increasing interest in one-carbon metabolism. Though the mechanism and the responsible metabolic pathway for the initiation and progression of one-carbon metabolism remain to be determined, impairments in folate-mediated one-carbon metabolism are associated with several common diseases and developmental anomalies including vascular disease, intestinal cancers, neural tube defects and cognitive decline. It is shown that folate deficiency plays a role in the development of neural tube defects. Other birth defects and adverse pregnancy outcomes are also shown to have something to do with the folate status. Folate deficiencies are primary causes of megaloblastic anemia. At the same time, abnormal folate status and high homocysteine level are considered to be the risk factors of colorectal cancer, depression, impaired cognitive function, cardiovascular diseases and other malignancies. While in vitro experiments indicate that high homocysteine may lead to vascular lesion, some but not all studies suggest that vascular dysfunction is at least partly due to accumulation of ADMA. Likewise, in one study, supplementation with folic acid or vitamin B12 could enhance cognition in the elderly.
It is without doubt that one-carbon metabolism play a significant in physiological and pathological process. The increasing interest in one-carbon metabolism in research filed and industrial field will demand powerful profiling analytical platform enabling the simultaneous determination of multiple components of one-carbon metabolism in large-scale studies. The compounds in one-carbon metabolism are of different chemical structure, various stability and variable polarity. To measure those compounds accurately and precisely with a single method is a true challenge. However, Creative Proteomics has established sensitive, reliable, and accurate HPLC-MS/MS method for quantification of one-carbon metabolism metabolites.
One-carbon Metabolism Analysis in Creative Proteomics
Folate and Folic Acid Analysis: This includes measuring folate levels in blood or tissues and assessing folic acid bioavailability and metabolism.
Methionine and Homocysteine Analysis: Quantifying methionine, a pivotal amino acid in one-carbon metabolism, and measuring homocysteine levels, which are influenced by this pathway.
Serum Vitamin B12 Analysis: Evaluating vitamin B12 status, crucial for facilitating one-carbon metabolism processes.
SAM (S-Adenosyl Methionine) and SAH (S-Adenosyl Homocysteine) Analysis: Quantifying SAM, a principal methyl donor, and assessing SAH levels, which reflect methylation potential and metabolic status.
Metabolomics Profiling: Comprehensive profiling of metabolites involved in one-carbon metabolism pathways, typically conducted using advanced techniques like LC-MS or NMR spectroscopy.
Dietary Assessment: Evaluating dietary intake of key nutrients crucial for one-carbon metabolism, such as folate, vitamin B12, and choline, to understand their impact on metabolic pathways.
Analytical Techniques for One-carbon Metabolism Analysis
Liquid Chromatography-Mass Spectrometry (LC-MS)
LC-MS combines the separating power of liquid chromatography with the sensitive detection capability of mass spectrometry. This technique separates metabolites based on their chemical properties and analyzes them based on their mass-to-charge ratio.
Applications:
- Metabolite Profiling: Quantification of key metabolites such as folates, S-adenosylmethionine (SAM), and related intermediates.
- Isotopic Labeling Studies: Tracking the incorporation of one-carbon units into metabolic pathways using stable isotopes.
- High-Throughput Analysis: Rapid screening of samples for comprehensive metabolic profiling.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS separates volatile and semi-volatile metabolites through gas chromatography and detects them using mass spectrometry. It is particularly useful for analyzing metabolites that are thermally stable and can be vaporized without decomposition.
Applications:
- Analysis of Amino Acids: Quantification of amino acids involved in one-carbon metabolism, such as glycine, serine, and methionine.
- Volatile Metabolites: Detection of small organic acids and other volatile compounds crucial in metabolic pathways.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy analyzes the magnetic properties of atomic nuclei within metabolites. It provides structural information and can elucidate dynamic changes in molecular environments.
Applications:
- Structural Elucidation: Determining the three-dimensional structure of metabolites involved in one-carbon metabolism.
- Interaction Studies: Investigating molecular interactions between metabolites and enzymes within the metabolic network.
Capillary Electrophoresis-Mass Spectrometry (CE-MS)
CE-MS separates charged metabolites based on their electrophoretic mobility in a capillary column and detects them using mass spectrometry. It offers high resolution and sensitivity for analyzing polar metabolites.
Applications:
- Quantitative Analysis: Accurate quantification of charged metabolites such as nucleotides and coenzymes involved in one-carbon metabolism.
- Metabolic Profiling: Comprehensive profiling of cellular metabolites to understand metabolic fluxes and regulatory mechanisms.
List of One-carbon Metabolism Metabolites We Can Analyze Includes, but is not limited to
| One-carbon Metabolism Metabolites Quantified in This Service | ||
|---|---|---|
| Betaine | Folate | Glycine |
| Glutamate | Homocysteine | L-Methionine |
| L-Serine | S-adenosylmethionine | Tetrahydrofolate |
| Vitamin B12 | ||
Sample Requirements for One-carbon Metabolism Analysis
| Sample Type | Minimum Sample Amount | Recommended Storage | Notes |
|---|---|---|---|
| Blood Plasma/Serum | 500 µL | Store at -80°C | Use EDTA or heparin as anticoagulant; avoid hemolysis |
| Tissue Samples | 50 mg | Snap-freeze in liquid nitrogen | Immediately after collection to preserve metabolites |
| Cell Cultures | 1 x 10^6 cells | Pellet and supernatant | Provide both for intracellular and extracellular analysis |
| Urine | 1 mL | Store at -80°C | Collect midstream urine; avoid repeated freeze-thaw cycles |
| CSF (Cerebrospinal Fluid) | 200 µL | Store at -80°C | Handle samples carefully to prevent contamination |
Important Considerations:
- Sample Collection: Follow standardized protocols for each sample type to ensure consistency and reliability of results.
- Storage Conditions: Maintain samples at -80°C immediately after collection to prevent degradation of metabolites.
- Sample Preparation: Remove contaminants and process samples according to specified guidelines to avoid assay interference.
- Handling: Minimize exposure to light and temperature fluctuations during sample handling and transportation.
- Documentation: Accurately label samples with identifiers and collection details to track sample integrity and provenance.

PCA chart

PLS-DA point cloud diagram

Plot of multiplicative change volcanoes

Metabolite variation box plot

Pearson correlation heat map
Cancer SLC43A2 alters T cell methionine metabolism and histone methylation
Journal: Nature
Published: 2020
Background
The study investigates the impact of methionine metabolism on T cell function within the tumor microenvironment. Methionine is crucial for SAM production, which influences histone methylation and epigenetic regulation in T cells. Dysfunctional T cells in tumors exhibit altered histone modifications, particularly H3K79me2, linked to methionine availability and SAM levels. Tumor cells, through the SLC43A2 transporter, competitively deplete methionine, potentially impairing T cell function and anti-tumor immunity.
Materials & Methods
Animal Models and Clinical Studies
Wild-type C57BL/6, BALB/c, and genetically modified mice (Dot1lflox/flox CD4-Cre) aged 6–12 weeks were used to investigate the impact of methionine and SAM depletion on T cell function. Mice were housed under specific pathogen-free conditions at the University of Michigan, following institutional guidelines. Clinical studies involved colorectal cancer patients recruited for a methionine supplementation trial, adhering to the Declaration of Helsinki with IRB approval from the Medical University of Lublin.
Reagents and Cell Culture
Amino acids including L-methionine, SAM, SAH, and metabolomics reagents were sourced from Sigma-Aldrich, Abcam, and other suppliers. RPMI 1640 medium without amino acids and specific inhibitors like α-(Methylamino) isobutyric acid (MeAIB) and 2-Amino-2-norbornanecarboxylic acid (BCH) were used for cell culture experiments. Methionine levels were quantified using the Methionine Assay Kit (Fluorometric, Abcam).
Cell Isolation and Culture
Primary human peripheral blood mononuclear cells (PBMCs) and mouse CD8+ T cells were isolated using Lymphoprep™ and EasySep™ kits, respectively. Human PBMCs were obtained from healthy donors, while mouse CD8+ T cells were isolated from spleen and lymph nodes of C57BL/6 mice. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), antibiotics, and specific amino acid concentrations or tumor cell supernatants.
Intracellular metabolites in CD8+ T cells and amino acids in human sera from healthy donors and ovarian cancer patients were quantified using LC-MS. Samples were processed by Creative-proteomics to analyze metabolic profiles and methionine concentrations in different experimental conditions.
Flow Cytometry and Molecular Analysis
Flow cytometry analysis was performed using BD LSRFortessa™ to assess cell surface markers (e.g., CD8, CD4), intracellular cytokines (IFNγ, TNFα), and apoptosis (Annexin V, 7-AAD). Molecular techniques included chromatin immunoprecipitation (ChIP) using antibodies against H3K79me2 (Abcam) to study histone modifications, and RNA sequencing (RNA-seq) to analyze gene expression profiles in CD8+ T cells exposed to varying methionine and SAM conditions.
Statistical Analysis
Data analysis was conducted using GraphPad Prism software (version 7). Statistical significance was determined using unpaired or paired two-tailed t-tests for inter-group comparisons, and two-way analysis of variance (ANOVA) for tumor growth experiments. Survival analysis was performed using Kaplan-Meier methods with log-rank tests. Correlations were assessed using Pearson correlation coefficients.
Results
Competitive Methionine Uptake in Tumor Microenvironment
We first demonstrated a direct competition between tumor cells and T cells for methionine, a critical substrate in one-carbon metabolism essential for SAM production. This competition resulted in decreased SAM levels in T cells, impacting their epigenetic landscape and functional phenotype.
Methionine Deprivation and Histone Modifications
Under conditions of methionine deprivation, T cells exhibited altered histone methylation patterns, particularly decreased levels of H3K79me2, a mark associated with active transcription. This reduction was attributed to the sensitivity of the methyltransferase DOT1L to SAM availability, highlighting methionine's regulatory role in epigenetic modifications crucial for T cell function.
 Tumor alters CD8+ T cell methionine metabolism to diminish H3K79me2
Tumor alters CD8+ T cell methionine metabolism to diminish H3K79me2
Impaired T Cell Function in Methionine-Deprived Environments
Functional assays revealed that T cells cultured in methionine-deficient conditions showed impaired cytokine production (e.g., IFNγ, TNFα) upon activation with anti-CD3 and anti-CD28 antibodies. Flow cytometry analysis further confirmed increased apoptosis rates among these T cells, indicating compromised viability and effector function.
Molecular Mechanisms of Methionine Sensitivity
Further investigation into molecular mechanisms revealed that methionine deficiency led to decreased STAT5 transcription factor activity in CD8+ T cells, associated with reduced H3K79me2 levels. This mechanistic link underscored the critical role of methionine metabolism in regulating transcriptional programs essential for T cell differentiation and function.
Metabolomic Profiling of Methionine-Deprived T Cells
Metabolomics analysis highlighted significant alterations in intracellular metabolite profiles of T cells deprived of methionine. LC-MS data showed distinct metabolic shifts, including reduced SAM levels and changes in amino acid concentrations, reflecting the metabolic adaptations driven by methionine availability in the tumor microenvironment.
 Tumor alters CD8+ T cell methionine metabolism to diminish H3K79me2.
Tumor alters CD8+ T cell methionine metabolism to diminish H3K79me2.
Preclinical Validation and Clinical Correlation
In vivo studies using mouse tumor models demonstrated that dietary methionine restriction impaired tumor growth and enhanced T cell-mediated anti-tumor immunity. Clinical studies in colorectal cancer patients supplemented with methionine further supported the role of methionine metabolism in modulating immune responses within the tumor milieu.
Reference
- Richter, Hadas, Ofer Gover, and Betty Schwartz. "Anti-inflammatory activity of black soldier fly oil associated with modulation of tlr signaling: A metabolomic approach." International Journal of Molecular Sciences 24.13 (2023): 10634.
What does "one carbon" mean in biochemistry?
In biochemistry, "one carbon" refers to a single carbon atom that serves as a fundamental building block for organic molecules. In one-carbon metabolism, these carbon units are transferred as methyl groups (-CH3), formyl groups (-CHO), or other derivatives. These transfers are crucial for the synthesis of amino acids, purines, and pyrimidines, which are essential for cellular growth, DNA replication, and maintenance of cellular functions. Proper regulation of one-carbon metabolism is vital for normal development, metabolism, and overall health.
What is the C1 metabolism pathway?
One-carbon (C1) metabolism refers to a complex network of biochemical reactions that manage the transfer and utilization of one-carbon units (methyl groups, formyl groups, etc.) in cells. These reactions are essential for synthesizing amino acids, nucleotides, and other important biomolecules. Key processes include the conversion of serine to glycine, the synthesis of thymidine from deoxyuridine monophosphate (dUMP), and the production of S-adenosylmethionine (SAM), a critical methyl donor for DNA methylation and various cellular processes.
What is the role of folate in one-carbon metabolism?
Folate, also known as vitamin B9, plays a central role in one-carbon metabolism by serving as a carrier of one-carbon units (methyl groups). Folate is converted to its active form, tetrahydrofolate (THF), which participates in reactions crucial for DNA synthesis, repair, and methylation. For instance, THF is involved in the conversion of homocysteine to methionine, a process essential for protein synthesis and cellular metabolism. Adequate folate intake is critical for normal cellular function and overall health.
What are the regulatory mechanisms of one-carbon metabolism enzymes?
Enzymes involved in one-carbon metabolism are tightly regulated to maintain cellular homeostasis and respond to metabolic demands. Regulation occurs through several mechanisms, including:
- Feedback inhibition: Downstream products inhibit upstream enzymes to prevent overproduction of metabolites.
- Gene expression control: Expression levels of enzymes are regulated by transcription factors in response to cellular signals and nutrient availability.
- Post-translational modifications: Enzyme activity can be altered by phosphorylation, acetylation, or other modifications in response to cellular needs.
- Subcellular localization: Enzymes may be compartmentalized within cells to control substrate availability and metabolic flux.
These regulatory mechanisms ensure that one-carbon metabolism adapts to changing metabolic conditions and supports cellular functions effectively.
Cancer SLC43A2 alters T cell methionine metabolism and histone methylation.
Bian, Y., Li, W., Kremer, D. M., Sajjakulnukit, P., Li, S., Crespo, J., ... & Zou, W.
Journal: Nature
Year:2020
https://doi.org/10.1038/s41586-020-2682-1
Metabolomic profiling implicates mitochondrial and immune dysfunction in disease syndromes of the critically endangered black rhinoceros (Diceros bicornis)
Corder, M. L., Petricoin, E. F., Li, Y., Cleland, T. P., DeCandia, A. L., Alonso Aguirre, A., & Pukazhenthi, B. S.
Journal: Scientific Reports
Year: 2023
https://doi.org/10.1038/s41598-023-41508-4
Transcriptomics, metabolomics and lipidomics of chronically injured alveolar epithelial cells reveals similar features of IPF lung epithelium
Willy Roque, Karina Cuevas-Mora, Dominic Sales, Wei Vivian Li, Ivan O. Rosas, Freddy Romero
Journal: bioRxiv
Year: 2020
https://doi.org/10.1101/2020.05.08.084459