What is the difference between SAM and SAH?
S-adenosylmethionine (SAM): The Universal Methyl Donor
SAM, or S-adenosylmethionine, is one of the most prominent co-substrates in cellular metabolism, functioning primarily as a methyl donor in a wide range of biochemical reactions. Formed through the reaction of ATP with the amino acid methionine, SAM acts as the principal methyl group donor for DNA, RNA, proteins, and various small molecules. The role of SAM in methylation reactions underpins its importance in several critical cellular processes:
- DNA methylation, which is vital for gene expression regulation, epigenetic modifications, and genomic stability.
- RNA methylation, which influences RNA stability, splicing, and translation.
- Protein methylation, especially in histones, which plays a major role in chromatin remodeling and gene transcription control.
Structurally, SAM comprises a sulfur group linked to methionine, which donates its methyl group in reactions catalyzed by methyltransferases. Once the methyl group is transferred, SAM is converted into SAH, marking the beginning of an intricate regulatory cycle.
S-adenosylhomocysteine (SAH): The Methylation Inhibitor
SAH, or S-adenosylhomocysteine, is formed as the by-product of methylation reactions that involve SAM. Structurally, SAH is similar to SAM but lacks the methyl group, which has been donated in the methylation process. Functionally, SAH acts as a potent negative regulator of methylation reactions, primarily because of its ability to inhibit methyltransferases.
This inhibition mechanism is critical in cellular homeostasis, as unchecked methylation could lead to deleterious effects, such as aberrant gene expression or protein misregulation. In this context, SAH plays a dual role—on one hand, it is the natural byproduct of methylation reactions, and on the other, its accumulation signals the cell to halt or modulate ongoing methylation processes.
Key Differences Between SAM and SAH
Function: SAM donates methyl groups in reactions, while SAH inhibits further methylation by accumulating as a byproduct.
Structure: SAM carries an active methyl group attached to the sulfur atom of methionine, whereas SAH lacks this methyl group post-reaction.
Biological Role: SAM drives essential methylation processes, while SAH regulates these processes by feedback inhibition, ensuring balanced cellular activity.
What is the SAM Cycle?
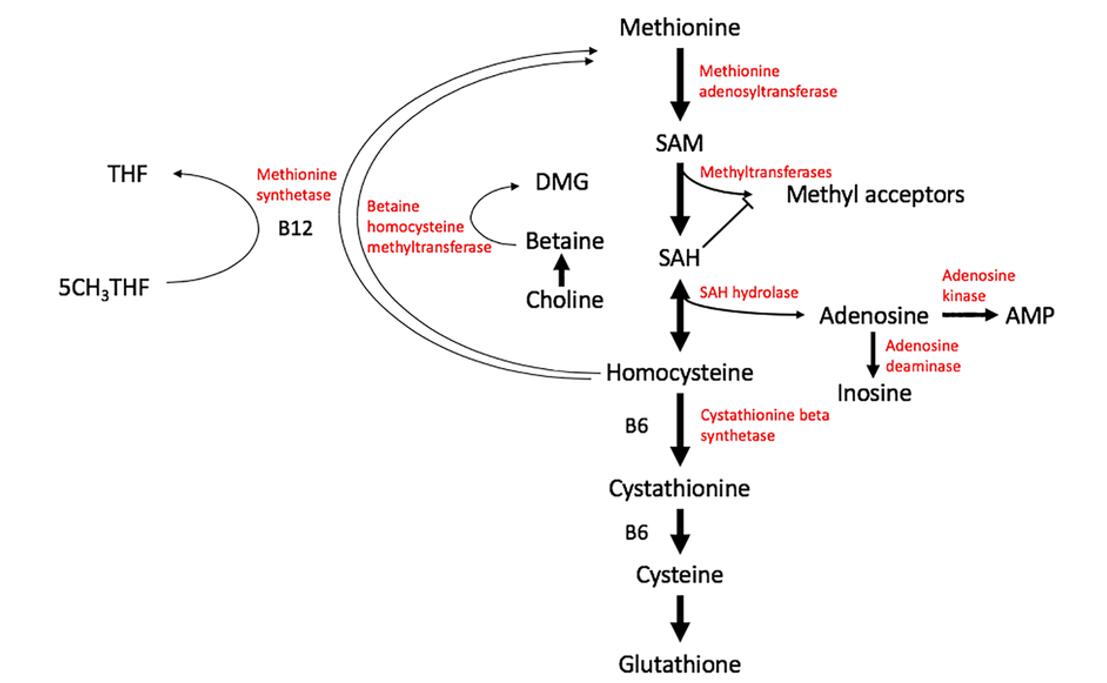
The SAM cycle, also known as the methylation cycle, is a fundamental biochemical pathway that governs the cellular supply of methyl groups, crucial for methylation reactions that regulate gene expression, protein function, and lipid metabolism. This cycle not only facilitates the synthesis and regeneration of S-adenosylmethionine (SAM) but also ensures the proper detoxification of its by-product, S-adenosylhomocysteine (SAH). The intricate coordination between methyl group donation and homocysteine recycling makes the SAM cycle a vital component of cellular homeostasis.
Formation and Role of SAM
The SAM cycle begins with the biosynthesis of SAM from methionine and adenosine triphosphate (ATP). This reaction is catalyzed by the enzyme methionine adenosyltransferase (MAT), which transfers the adenosyl moiety of ATP to the sulfur atom of methionine, forming SAM. This step is highly conserved across species, reflecting its central importance in cellular metabolism. SAM is often termed the "universal methyl donor", given its pivotal role in transferring methyl groups (-CH3) to various substrates, including DNA, RNA, proteins (such as histones), and small molecules like neurotransmitters and phospholipids.
Methylation Reactions: SAM as a Methyl Donor
SAM's principal role in the cycle is to serve as a donor of methyl groups in reactions catalyzed by methyltransferases. During these reactions, SAM donates its methyl group to acceptor molecules, which may include cytosine residues in DNA (for DNA methylation), nitrogenous bases in RNA (for RNA methylation), lysine or arginine residues in histone proteins (for histone methylation), and other small molecules. This methyl group transfer is critical for epigenetic regulation, influencing gene expression patterns, chromatin structure, and signal transduction pathways.
Once SAM donates its methyl group, it is converted into S-adenosylhomocysteine (SAH), a reaction product that serves as a key regulatory metabolite in the methylation cycle.
SAH as a Methylation Inhibitor and Its Hydrolysis
The generation of SAH from SAM is not a passive outcome but rather a critical regulatory point within the cycle. SAH, when accumulated, acts as a competitive inhibitor of methyltransferases, effectively halting further methylation reactions. This negative feedback mechanism ensures that excessive methylation does not occur, protecting the cell from potential dysregulation of gene expression or protein function. Therefore, the timely removal of SAH from the intracellular environment is vital for maintaining methylation efficiency.
The enzyme S-adenosylhomocysteine hydrolase (SAHH) catalyzes the hydrolysis of SAH into two distinct metabolites: homocysteine and adenosine. This reaction is reversible under certain conditions, although it generally proceeds in the direction of SAH hydrolysis to prevent SAH accumulation and its inhibitory effects on methyltransferases. The role of SAHH in the SAM cycle is crucial, as the accumulation of SAH would severely impede methylation capacity.
Recycling of Homocysteine: Methionine Remethylation and Transsulfuration
The homocysteine produced from SAH hydrolysis can follow two distinct metabolic fates within the cell: remethylation back to methionine or diversion into the transsulfuration pathway.
Remethylation to Methionine: To sustain the cycle and replenish methionine for SAM synthesis, homocysteine must be remethylated. This is primarily achieved through two key enzymes: Methionine synthase (MS), a vitamin B12-dependent enzyme, catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate (a derivative of folate) to homocysteine, regenerating methionine.Alternatively, in liver and kidney cells, betaine-homocysteine methyltransferase (BHMT) can also catalyze this remethylation reaction, using betaine as the methyl donor.
This remethylation process is tightly linked to the folate cycle, where folate-derived compounds provide the necessary methyl groups for methionine regeneration. The efficient remethylation of homocysteine ensures a continuous supply of methionine for SAM synthesis, allowing the cycle to proceed unimpeded.
Transsulfuration Pathway: When cellular levels of SAM are high, homocysteine is directed into the transsulfuration pathway rather than being remethylated. This pathway, initiated by cystathionine β-synthase (CBS), a vitamin B6-dependent enzyme, converts homocysteine into cystathionine, which is subsequently broken down into cysteine. Cysteine is a precursor for glutathione, one of the cell's primary antioxidants, linking the SAM cycle to redox homeostasis and oxidative stress responses. This diversion into the transsulfuration pathway allows the cell to modulate homocysteine levels according to the demand for methylation or sulfur-containing metabolites.
Regulation of the SAM Cycle
The SAM cycle is tightly regulated to balance methylation demands with methionine and homocysteine metabolism. Several factors contribute to this regulation:
- Allosteric regulation by SAM: SAM serves as an allosteric activator of cystathionine β-synthase (CBS), promoting the transsulfuration pathway when SAM levels are elevated. This ensures that excess SAM is not continuously recycled into methylation reactions, preventing hypermethylation.
- Nutrient availability: The availability of cofactors such as folate (vitamin B9), vitamin B12, and vitamin B6 is critical for maintaining the proper flow through the SAM cycle. Deficiencies in these vitamins can impair methionine remethylation and lead to elevated homocysteine levels, a condition known as hyperhomocysteinemia, which is associated with cardiovascular disease, neurodegenerative disorders, and other health issues.
- Feedback inhibition by SAH: The inhibitory effect of SAH on methyltransferases serves as an intrinsic feedback mechanism within the cycle, preventing excessive methylation and ensuring that SAM-dependent reactions occur in a controlled manner. SAHH's role in hydrolyzing SAH is thus pivotal for maintaining methylation flux and ensuring the balance between SAM utilization and SAH detoxification.
Physiological Implications of the SAM Cycle
The SAM cycle is not an isolated metabolic pathway but rather a central node connecting multiple metabolic and regulatory networks. Its influence extends across:
- Epigenetic regulation: DNA and histone methylation are directly dependent on SAM availability, linking the SAM cycle to gene expression control. Changes in the SAM/SAH balance can result in altered epigenetic landscapes, affecting cellular differentiation, proliferation, and adaptation.
- Cellular redox balance: By driving homocysteine into the transsulfuration pathway, the SAM cycle contributes to glutathione synthesis, which is essential for protecting the cell against oxidative damage. This cross-talk between methylation and redox regulation underscores the cycle's importance in cellular stress responses.
- Neurotransmitter synthesis: SAM is involved in the methylation of neurotransmitters such as norepinephrine and serotonin, implicating the SAM cycle in neurochemical homeostasis. Disruptions in SAM metabolism have been linked to neurological and psychiatric disorders, further highlighting the cycle's systemic relevance.
Dysregulation of the SAM Cycle and Disease
Impairments in the SAM cycle can have far-reaching consequences for cellular health. Elevated homocysteine levels due to disruptions in remethylation or transsulfuration pathways are associated with increased cardiovascular risk, while reduced SAM levels may lead to insufficient methylation, contributing to aberrant gene expression, carcinogenesis, and neurodegeneration. Understanding the precise regulation of the SAM cycle is thus critical not only for basic metabolic processes but also for therapeutic interventions in diseases where methylation imbalances are a key factor.
Learn more about SAM and SAH Analysis Service.
What does SAM/SAH ratio mean?
The SAM/SAH ratio is a key metabolic indicator reflecting the cell's capacity for methylation reactions. It is often referred to as the "methylation index", as it provides insights into the cell's methylation potential at any given time.
- A high SAM/SAH ratio suggests an abundance of SAM relative to SAH, indicating robust methylation capacity. This condition is generally associated with healthy cellular metabolism where methylation processes are functioning optimally.
- A low SAM/SAH ratio suggests that SAH levels are relatively high compared to SAM. This can lead to the inhibition of methyltransferases, effectively reducing the cell's ability to carry out essential methylation reactions.
Biological and Clinical Implications
The SAM/SAH ratio is crucial in multiple biological contexts:
Epigenetic Regulation: Since methylation is a key mechanism in epigenetic modifications, the SAM/SAH ratio directly affects gene expression patterns. A balanced ratio supports normal epigenetic regulation, while an imbalanced ratio can contribute to abnormal gene silencing or activation.
Disease States: A low SAM/SAH ratio has been linked to pathological conditions, including cancer, cardiovascular diseases, and neurodegenerative disorders. In many cases, these diseases are characterized by disrupted methylation patterns, either through hypermethylation or hypomethylation, driven by altered SAM/SAH ratios.
Cellular Stress and Aging: As cells age or experience oxidative stress, methylation capacity often diminishes, which can be reflected in a declining SAM/SAH ratio. Restoring this ratio through dietary interventions or supplementation (e.g., folate, B-vitamins) has been a topic of interest in therapeutic strategies aimed at mitigating age-related decline in cellular function.
Regulating the SAM/SAH Ratio
The SAM/SAH ratio is influenced by several factors, including:
- Diet: Nutrients such as folate, vitamin B12, and methionine directly affect the SAM cycle, influencing the balance between SAM and SAH. Insufficient intake of these nutrients can impair methylation processes.
- Metabolic Health: Conditions such as hyperhomocysteinemia, characterized by elevated homocysteine levels, can disrupt the SAM cycle and lower the SAM/SAH ratio. Proper metabolic regulation of homocysteine is essential for maintaining methylation capacity.
- Therapeutic Interventions: Strategies to modulate the SAM/SAH ratio include the use of SAM-e supplements (to boost SAM levels) or folate and B-vitamin supplementation to promote methionine remethylation, thereby restoring methylation potential.
Reference
Chang, Caitlin A., et al. "Transiently elevated plasma methionine, S‐adenosylmethionine and S‐adenosylhomocysteine: unreported laboratory findings in a patient with NGLY1 deficiency, a congenital disorder of deglycosylation." JIMD reports 49.1 (2019): 21-29.