Pyrimidine metabolism is a fundamental biochemical pathway that plays an essential role in the synthesis and degradation of nucleotides, the building blocks of DNA and RNA. Nucleotides derived from pyrimidines, such as cytosine (C), uracil (U), and thymine (T), are crucial for genetic replication, transcription, and cell division. Without pyrimidines, cells would be unable to proliferate or maintain their genomic integrity, and by extension, organisms would not survive.
Understanding the intricacies of pyrimidine metabolism not only provides insight into cellular biology but also has direct implications for human health. Aberrations in these pathways are linked to several genetic disorders, and targeting pyrimidine metabolism has become a focal point in developing therapies for cancer and other proliferative diseases.
Pyrimidine Synthesis Pathways
Pyrimidines can be synthesized by cells in two distinct ways: the de novo pathway and the salvage pathway. Both are essential, but the pathways differ in how they generate pyrimidines and in the circumstances under which they are activated.
De Novo Synthesis Pathway
The de novo pathway synthesizes pyrimidine nucleotides from simple molecules like bicarbonate, aspartate, and glutamine. This pathway is highly energy-intensive, consuming ATP, and is tightly regulated to match the cellular demand for nucleotides.
The key steps in de novo pyrimidine synthesis include:
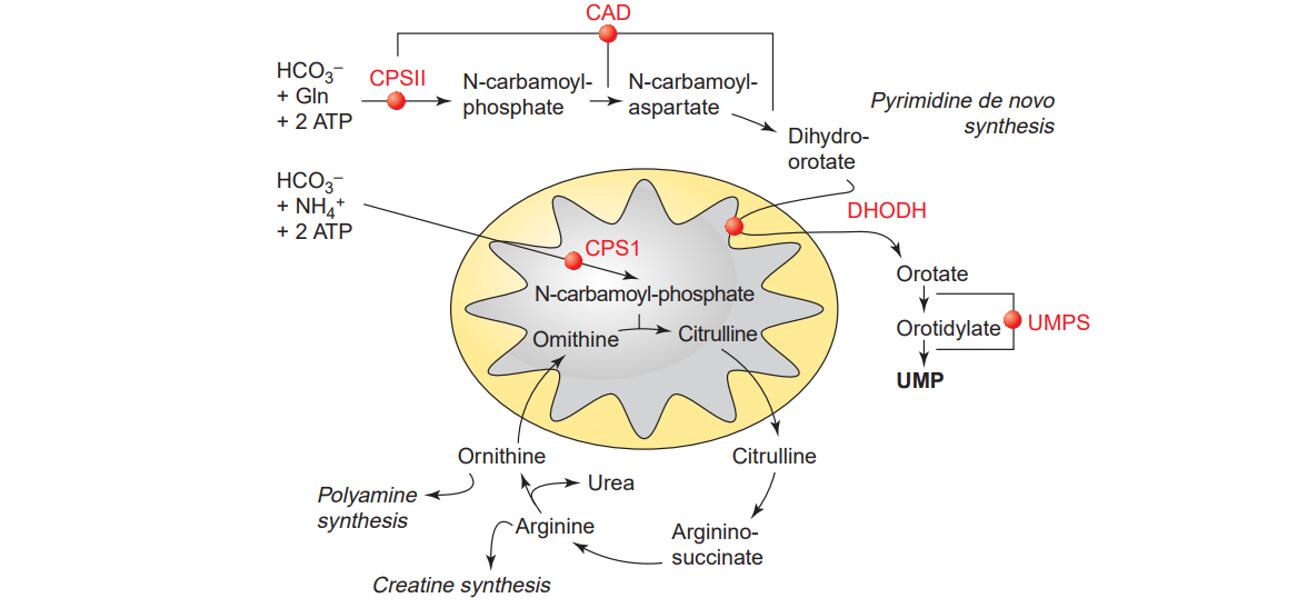
- Formation of Carbamoyl Phosphate: The enzyme carbamoyl phosphate synthetase II (CPS II) catalyzes the first rate-limiting step, converting glutamine, CO₂, and ATP into carbamoyl phosphate. This step is a critical regulatory point and is subject to feedback inhibition by UTP.
- Conversion to Orotic Acid: In a series of reactions, carbamoyl phosphate reacts with aspartate to form carbamoyl aspartate, which is then cyclized to produce dihydroorotate. This is subsequently oxidized by the enzyme dihydroorotate dehydrogenase (DHODH) to form orotic acid.
- Formation of UMP: Orotic acid undergoes a phosphoribosylation step to form orotidine monophosphate (OMP). The enzyme OMP decarboxylase then catalyzes the removal of a carboxyl group to yield uridine monophosphate (UMP), the first pyrimidine nucleotide produced in the de novo pathway.
Once UMP is formed, it can be phosphorylated to form UDP and UTP, which can be further converted into other pyrimidine nucleotides, including CTP and, in the case of DNA synthesis, dTTP.
Salvage Pathway
The salvage pathway allows cells to recycle free pyrimidine bases and nucleosides derived from nucleotide degradation. This pathway is less energy-intensive and is especially critical in tissues with low proliferative activity, where de novo synthesis is not as active.
Key reactions in the salvage pathway involve enzymes like:
- Uridine phosphorylase and thymidine kinase, which recover uracil, thymine, and cytosine bases, converting them back into nucleotides.
The balance between de novo synthesis and the salvage pathway is vital for maintaining an adequate supply of pyrimidines while conserving cellular energy resources.
Pyrimidine Degradation Pathways
Pyrimidine degradation is a crucial biochemical process responsible for breaking down the pyrimidine nucleotides—cytosine, uracil, and thymine—into simpler, non-toxic metabolites that can either be further utilized or excreted from the body. Unlike purine degradation, which leads to the production of uric acid (a metabolite that can cause pathological conditions such as gout if accumulated in excess), the breakdown of pyrimidines results in water-soluble end products that do not pose a significant health risk under normal physiological conditions. The efficiency and regulation of pyrimidine degradation are essential for maintaining cellular nucleotide balance and preventing toxic accumulation of intermediates.
Key Steps in Pyrimidine Degradation
Pyrimidine degradation primarily occurs in the liver, where specialized enzymes facilitate the stepwise breakdown of pyrimidine nucleotides into smaller components. These end products can be repurposed for other metabolic pathways or safely removed from the body.
Cytosine and Uracil Catabolism
The degradation of cytosine and uracil follows a similar pathway. Cytosine is first deaminated by cytosine deaminase, converting it into uracil by removing an amine group. Uracil is then further broken down through a multi-step process:
- Reduction of Uracil: The enzyme dihydropyrimidine dehydrogenase (DPD) initiates the degradation by reducing uracil to dihydrouracil. This is a key regulatory step, and DPD is subject to genetic variability, influencing how efficiently individuals process pyrimidines. Notably, DPD activity plays a role in drug metabolism, particularly for chemotherapeutics like 5-fluorouracil.
- Ring Cleavage: Following reduction, dihydrouracil is opened by dihydropyrimidinase, splitting the pyrimidine ring structure. This step results in the formation of β-ureidopropionate.
- Final Breakdown: The final step in uracil degradation involves β-ureidopropionase, which cleaves β-ureidopropionate into β-alanine, CO₂, and ammonia (NH₃). β-alanine can be further utilized in the synthesis of other biomolecules, such as coenzyme A (CoA), or excreted as a waste product.
Thymine Catabolism
Thymine, which differs from uracil by the presence of a methyl group, undergoes a parallel degradation pathway but yields different final products:
- Reduction of Thymine: Like uracil, thymine is reduced to dihydrothymine by dihydropyrimidine dehydrogenase (DPD). This reduction step is again critical for regulating thymine breakdown and ensuring the proper removal of excess nucleotides from the body.
- Ring Cleavage and Decomposition: Dihydrothymine is then cleaved by dihydropyrimidinase, forming β-ureidoisobutyrate, which is subsequently acted upon by β-ureidopropionase. The end products of thymine degradation are β-aminoisobutyric acid (β-AIB), CO₂, and ammonia. β-AIB is a unique marker of thymine degradation and can be measured in urine as an indicator of DNA turnover and cell death, particularly in cases of rapid cell division or apoptosis.
Biological Significance of Pyrimidine Degradation
The degradation of pyrimidines serves several critical functions in maintaining cellular homeostasis:
Detoxification and Waste Removal: Pyrimidine catabolism prevents the accumulation of excess nucleotides and their potentially toxic intermediates. By breaking them down into non-toxic, water-soluble products like β-alanine and β-AIB, the body ensures efficient excretion through urine. This is particularly important in rapidly dividing tissues, where nucleotide synthesis and turnover are high.
Recycling and Metabolic Integration: The final degradation products of pyrimidines, such as β-alanine and β-AIB, are not simply waste products. These metabolites can be recycled for use in other important biological processes. For instance, β-alanine is a precursor in the biosynthesis of pantothenic acid (vitamin B5), which is essential for the production of coenzyme A, a cofactor involved in numerous metabolic reactions, including fatty acid oxidation and the citric acid cycle.
Metabolic Flexibility: Pyrimidine degradation integrates with broader metabolic networks. The catabolic pathways of cytosine, uracil, and thymine feed into amino acid metabolism and energy production. The conversion of β-ureidopropionate and β-ureidoisobutyrate into simpler compounds allows cells to efficiently manage their energy reserves and nutrient utilization.
Regulation of Pyrimidine Metabolism
The regulation of pyrimidine metabolism is a sophisticated and dynamic process, essential for maintaining cellular homeostasis and ensuring that nucleotide pools are balanced according to cellular needs. This regulation is tightly intertwined with the cell's proliferative state, energy availability, and overall demand for nucleotides, which fluctuate depending on factors like growth, division, and environmental stimuli. Two major regulatory mechanisms—feedback inhibition and cell cycle-dependent control—serve to finely tune the synthesis and degradation of pyrimidine nucleotides, preventing unnecessary energy expenditure while ensuring that nucleotides are available for critical processes such as DNA replication and repair.
Feedback Inhibition in Pyrimidine Metabolism
The de novo pyrimidine synthesis pathway is stringently regulated through feedback inhibition, a form of allosteric control that helps cells avoid overproduction of nucleotides when supplies exceed demand. This ensures that pyrimidine metabolism operates efficiently, conserving energy and substrates.
CPS II (Carbamoyl Phosphate Synthetase II) Regulation:
CPS II catalyzes the first and most critical committed step in de novo pyrimidine synthesis, combining bicarbonate, glutamine, and ATP to form carbamoyl phosphate. CPS II is subject to intricate regulation, acting as a key control point:
- Inhibition by UTP: When levels of UTP (uridine triphosphate) are sufficient within the cell, UTP binds allosterically to CPS II, reducing its activity. This feedback inhibition prevents the over-accumulation of pyrimidine nucleotides, ensuring that the pathway is downregulated when pyrimidine pools are already adequate.
- Activation by PRPP: On the other hand, CPS II is activated by high levels of phosphoribosyl pyrophosphate (PRPP), a precursor in nucleotide biosynthesis. Elevated PRPP signals that the cell requires more nucleotides for growth and division, prompting CPS II to ramp up its activity to meet this increased demand.
CTP Synthetase Regulation:
CTP synthetase is another key enzyme in pyrimidine metabolism that converts UTP to CTP (cytidine triphosphate), a necessary step for RNA and lipid biosynthesis. CTP synthetase is negatively regulated by its product, CTP, through feedback inhibition. This mechanism ensures that when intracellular CTP concentrations are high, further synthesis is curtailed, thus preventing an unnecessary buildup of cytidine nucleotides. By modulating CTP production, the cell balances its RNA synthesis requirements with its overall pyrimidine pool, maintaining nucleotide homeostasis while preventing metabolic waste.
This type of allosteric regulation, common in metabolic pathways, helps ensure that the cell produces only the nucleotides it needs, sparing energy and resources when pyrimidine levels are sufficient. It also prevents the toxic effects of nucleotide imbalances, which could disrupt cellular functions like DNA and RNA synthesis or lead to uncontrolled cell proliferation.
Cell Cycle-Dependent Control
Pyrimidine metabolism is intricately linked to the cell cycle, particularly to the needs of cells as they prepare for division. As cells progress through the cell cycle, their requirement for nucleotides fluctuates, especially during the DNA synthesis phase (S-phase), when large quantities of pyrimidine nucleotides are required for the replication of genetic material.
S-Phase Upregulation of Pyrimidine Synthesis:
During the S-phase of the cell cycle, cells must replicate their entire genome, which dramatically increases the demand for nucleotides, including pyrimidines like UTP, CTP, and thymidine triphosphate (TTP). To meet this demand, the cell upregulates the expression and activity of key enzymes involved in both pyrimidine and purine synthesis. For instance:
- Ribonucleotide reductase, the enzyme responsible for converting ribonucleotides to deoxyribonucleotides (the building blocks of DNA), is heavily upregulated in S-phase. This ensures that sufficient quantities of dUTP, dCTP, and dTTP are available for DNA replication.
- Enzymes like CPS II and UMP synthase are also activated during this phase, further driving the de novo synthesis of pyrimidine nucleotides to replenish the cellular nucleotide pool as DNA replication proceeds.
Coordination with Other Metabolic Pathways:
The increased demand for pyrimidines during the cell cycle also requires coordination with other metabolic pathways. The production of PRPP, which is essential for both de novo and salvage pathways of nucleotide synthesis, is upregulated in tandem with pyrimidine synthesis to ensure a sufficient supply of precursors. Additionally, energy metabolism is closely coupled with pyrimidine metabolism during the cell cycle. As pyrimidine synthesis is an ATP-consuming process, cells enhance their energy production to support the heightened biosynthetic activity necessary for cell division.
Regulation by Cell Cycle Checkpoints:
The synthesis of pyrimidines is also controlled by cell cycle checkpoints, which monitor DNA integrity and nucleotide availability before allowing progression through critical stages of the cell cycle. If pyrimidine pools are insufficient, or if there is DNA damage that requires repair, checkpoints can delay cell cycle progression, allowing time for pyrimidine synthesis or DNA repair to occur. This ensures that cells do not enter DNA replication with inadequate nucleotide supplies, which could lead to incomplete or erroneous DNA replication and result in genomic instability.
Transcriptional Control:
In addition to enzyme regulation, pyrimidine metabolism is also influenced at the transcriptional level. Certain genes involved in pyrimidine biosynthesis, such as those encoding thymidylate synthase or dihydroorotate dehydrogenase, are upregulated during periods of active cell division. This transcriptional regulation ensures that the enzymes necessary for pyrimidine synthesis are available in sufficient quantities at the right time in the cell cycle, aligning nucleotide production with the cell's proliferative needs.
Clinical Implications and Disorders of Pyrimidine Metabolism
The importance of pyrimidine metabolism is underscored by the fact that disruptions in this pathway can lead to serious health issues. Some of the most significant clinical implications include genetic disorders and the therapeutic targeting of pyrimidine metabolism.
Genetic Disorders
Several rare genetic disorders arise from mutations in enzymes involved in pyrimidine metabolism:
- Orotic Aciduria: A deficiency in OMP decarboxylase causes orotic acid to accumulate, leading to growth retardation and anemia. This condition can be treated with oral uridine, which bypasses the metabolic block.
- Dihydropyrimidine Dehydrogenase (DPD) Deficiency: Mutations in the DPYD gene result in impaired degradation of uracil and thymine. This can lead to developmental delays, seizures, and other neurological symptoms. Moreover, DPD deficiency has significant implications in cancer therapy, as it affects the metabolism of certain chemotherapy drugs like 5-fluorouracil (5-FU).
Cancer and Therapeutic Targeting
Pyrimidine metabolism is also a target for cancer therapies. Many rapidly dividing cancer cells rely heavily on the de novo pyrimidine synthesis pathway to meet their high nucleotide demands. As a result, several chemotherapy agents target key enzymes in this pathway:
- 5-Fluorouracil (5-FU): This drug inhibits thymidylate synthase, which is essential for the conversion of dUMP to dTMP, ultimately blocking DNA synthesis in cancer cells.
- Leflunomide: Used in treating rheumatoid arthritis and some cancers, leflunomide inhibits dihydroorotate dehydrogenase (DHODH), thus preventing pyrimidine synthesis and cell proliferation.
By targeting pyrimidine metabolism, these therapies exploit the unique vulnerabilities of cancer cells, offering a strategic approach to cancer treatment.
Reference
Löffler, Monika, et al. "Pyrimidine pathways in health and disease." Trends in molecular medicine 11.9 (2005): 430-437.