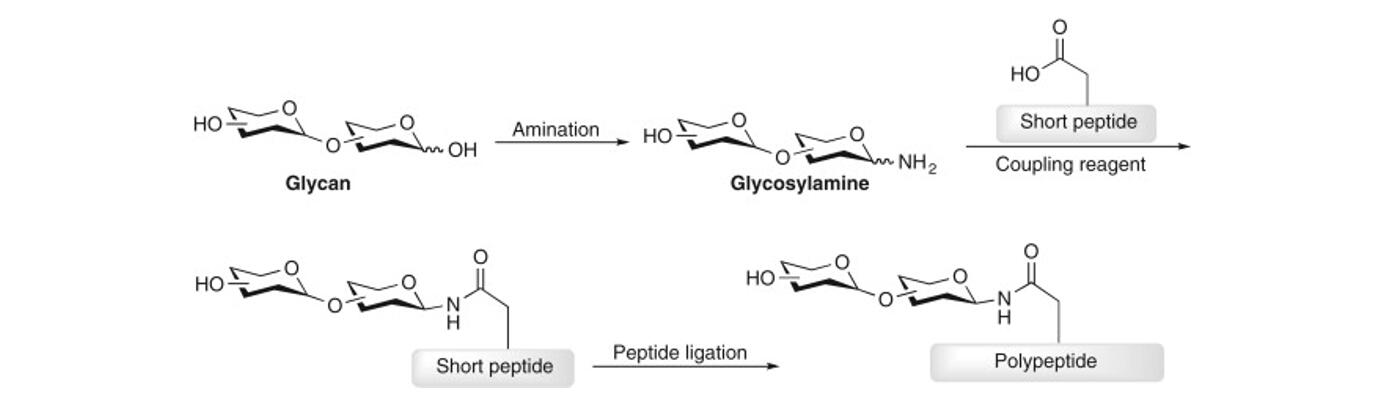
Protein glycosylation is a very common, complex and diverse post-translational modification of proteins. In eukaryotes, protein glycosylation process mainly occurs within the endoplasmic reticulum and Golgi system, involving about 200 glycosyltransferases and causing 50%-70% of proteins to be modified by glycosylation. According to the different ways of glycan and protein linkage, protein glycosylation is mainly classified into 4 major types: N-glycosylation, O-glycosylation, C-glycosylation and glycosylphosphatidylinositol (GPI) anchoring. N- and O-glycosylation have been most extensively studied.
In N-glycosylation, the sugar chain is covalently bound to the asparagine (Asn) side chain amino group via N-acetylglucosamine (GlcNAc). The sugar chain has a pentasaccharide core structure and the glycosylation site has a specific peptide sequence Asn-X-Ser/Thr (X ≠ Pro). In O-glycosylation, the sugar chain is covalently bound by hydroxyl groups of the side chain with serine (Ser) or threonine (Thr). There is no fixed core structure of the glycan chain and no conserved peptide sequence at the glycosylation site. N- and O-glycosylation plays an important role in protein stability, intracellular and intercellular signaling, hormone activation or inactivation, and immune regulation. Abnormal expression of protein glycosylation is also often indicative of the development of related diseases, such as inflammation, cancer, and genetic disorders.
Glycopeptides are a class of macromolecular compounds that combine oligosaccharides with peptides. Glycopeptides have important applications in the development of glycopeptide drugs, such as glycopeptide antibiotics and antitumor vaccines.
Currently, most of the tumor biomarkers approved for clinical use are glycoproteins. For example, alpha-fetoprotein (AFP) for liver cancer, cancer antigen 125 (CA125) for ovarian cancer, carcinoembryonic antigen (CEA) for colon cancer, and prostate-specific antigen (PSA) or glycoantigen for prostate cancer. Therefore, glycoproteomic analysis is important for early screening of diseases and pathological studies, etc.
Mass spectrometry-based proteomics techniques provide an excellent analytical tool for comprehensive analysis of proteins and their modifications. In bottom-up proteomics, whole proteins of single proteins or complex biological samples (cells, tissues, body fluids, etc.) are usually digested enzymatically into peptides before being identified and quantified by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS).
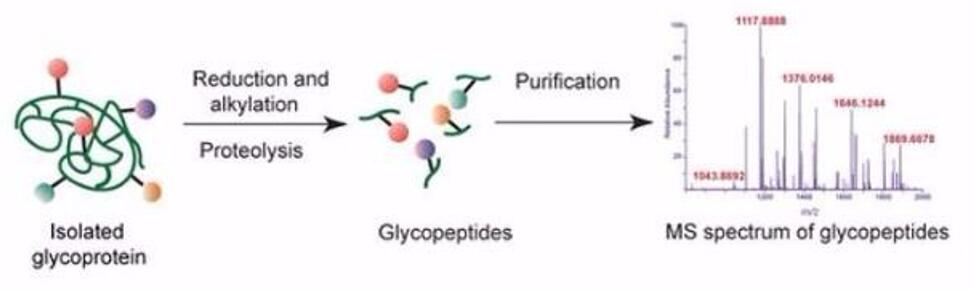
Schematic diagram of the steps involved in glycopeptides analysis
Comprehensive analysis of protein glycosylation remains challenging due to the diversity and high heterogeneity of glycan chains. Despite the prevalence of protein glycosylation, the low abundance of glycoproteins relative to whole proteins and the even lower abundance of intact glycopeptides make it difficult to perform direct detection by mass spectrometry. Therefore, in order to increase the abundance of intact glycopeptides in complex biological samples, it is important to develop enrichment methods for intact glycopeptides. At present, the common glycopeptide enrichment techniques include hydrophilic interaction chromatography, metal affinity chromatography, lectin affinity chromatography, boronic acid chemistry, enzyme chemistry, hydrazide chemistry, etc.
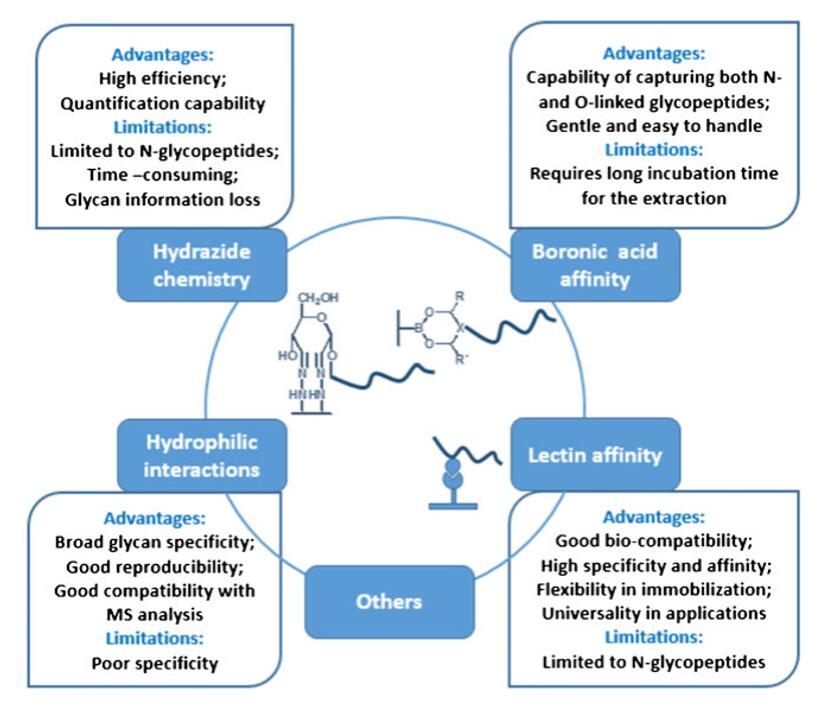
Experimental strategies for glycoprotein/glycopeptide enrichment (Li et al., 2014)
The low ionization efficiency of glycopeptides and the high heterogeneity of glycan chains compared to non-modified peptides make the mass spectrometric characterization of intact glycopeptides challenging. Therefore, it is important to develop effective mass spectrometry fragmentation methods and spectral resolution tools for the comprehensive analysis of intact glycopeptides. In the last decade, with the innovation of mass spectrometry technology, the quality of spectral data is getting higher and higher, and the software algorithms for accurate resolution of intact N- and O-glycopeptides have been developed rapidly.
Creative Proteomics can provide you with glycopeptide analysis services. We use pre-MS separation procedures and HILIC columns for LC-MS separation of glycopeptides.
Reference
Li, L., Xu, L., Li, Z., Bai, Y., & Liu, H. (2014). Novel nanomaterials used for sample preparation for protein analysis. Analytical and bioanalytical chemistry, 406(1), 35-47.