Immunoglobulin is a serum glycoprotein. There are five types of IgM, IgA, IgD, IgG and IgE found in humans. IgG consists of two Fab segments and one Fc segment, while Fab specifically binds to recognized antigens. Fc can bind to different Fc receptors or complement to mediate its effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP) or complement dependent cytotoxicity (CDC).
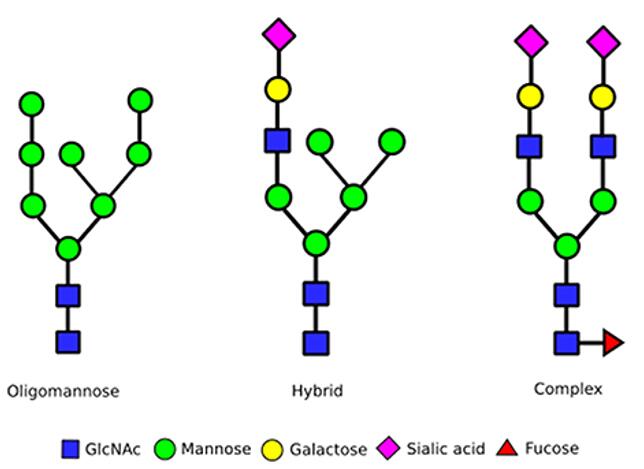
The N-glycosylation of serum IgG is located at a common sequence in the CH2 region of the Fc fragment (Asn297-X-Ser/Thr, X is any amino acid residue except proline), which is covalently bound to the antibody via an amide bond. Thirty percent of them also have an N-glycosylation site in Fab.
The glycosylation of serum antibodies is a complex biallelic glycan type and may have a branching N-acetylglucosamine structure. The vast majority of monoclonal antibodies are expressed by CHO, SP2/0 and NS0 cells, and most of them only have an N-glycosylation site at approximately 297 positions on the heavy chain Fc (except for cetuximab) and do not contain a branching N-acetylglucosamine structure.
So what is the function of N-glycosylation in monoclonal antibodies?
The structure of N-glycans maintains the conformation of monoclonal antibodies and affects Fc effector function
The IgG-Fc crystal structure shows that the oligosaccharide is wrapped in two CH2 structural domains and there are multiple hydrophobic interactions between the glycoform structure and the Fc structural domain. The G0 glycoform has 52 interactions with the monoclonal Fc Cγ2 structural domain, the core fucose has 7 interactions, and the galactose on the α1-6 arm has 27 interactions. It is noteworthy that the sugar on the α1-3 arm does not contact the surface of the monoclonal antibody, but penetrates deeply into the space formed by the two heavy chains of Fc. The interaction between the mannose on the α1-3 arm of the two sugar chains is important for maintaining the conformation of the monoclonal antibody. Without the presence of the glycans, the CH2 domain of the Fc is slightly enlarged, resulting in a slightly earlier elution time in molecular exclusion chromatography, a more sensitive and aggregation-prone behavior in thermal acceleration experiments, and a loss of the ADCC and CDC effects, as well as an increased sensitivity to proteases and instability.
Relationship between N-glycan structure and ADCC and ADCP
The N-glycan on Fc can contain core fucose transferred to the core N-acetylglucosamine by α1-6 fucosyltransferase. The deletion of core fucose could significantly enhance the binding of monoclonal antibody Fc to FcγRIIIa, leading to a significant increase in its ADCC effect. Sialic acid on the N-glycan terminus of Fc could weaken the ADCC effect of monoclonal antibodies, probably because the binding of monoclonal antibodies Fc to FcγRIIIa is weakened by high sialylation.
Relationship between N-glycan structure and CDC
The N-glycan terminus on Fc may contain 0, 1 or 2 terminal Gal residues (G0, G1 or G2), and the terminal Gal content increases the CDC effect by increasing the binding of antibody and C1q, but does not affect the binding of antibody and FcγRIIIa, so it has no effect on ADCC activity. The CDC effect of monoclonal antibodies containing high-mannose type N-glycans on Fc is also reduced, which may be due to the fact that high-mannose type N-glycans do not contain galactose at their ends.
Analysis of N-glycans
Monoclonal antibody products require thorough analysis of the glycoforms. Currently, protein n-glycosylation can be analyzed by mass spectrometry at the level of intact glycoproteins or protein subunits. Intact protein level analysis provides information on the molecular weight of the protein and the relative content of the different glycoforms. Analysis of the reduced heavy chain provides more accurate information on glycoforms and other post-translational modifications such as lysine deletion and oxidation. Analysis can also be performed at the glycopeptide level to obtain information on glycosylation modification sites. This method is usually used when a protein contains multiple glycosylation sites.
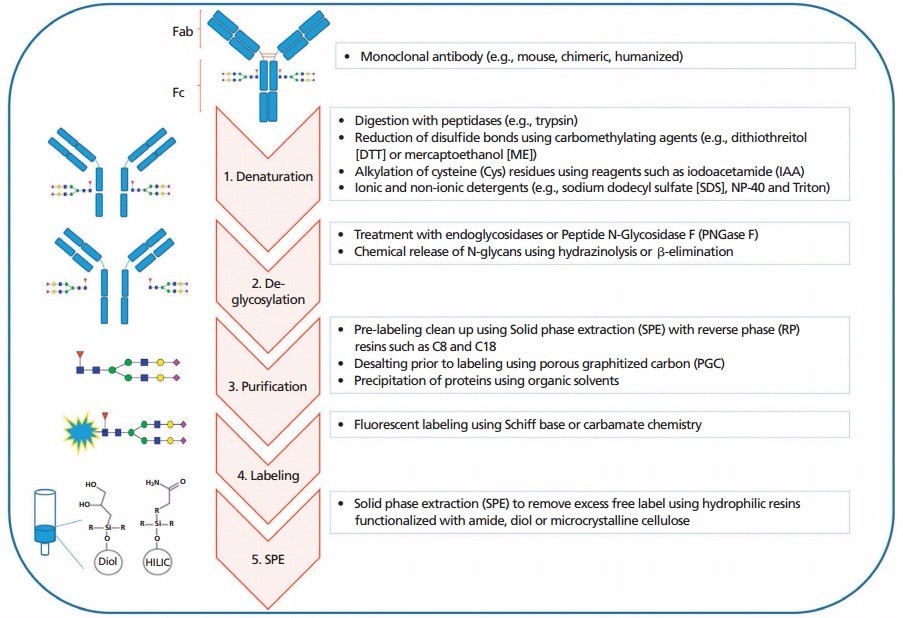
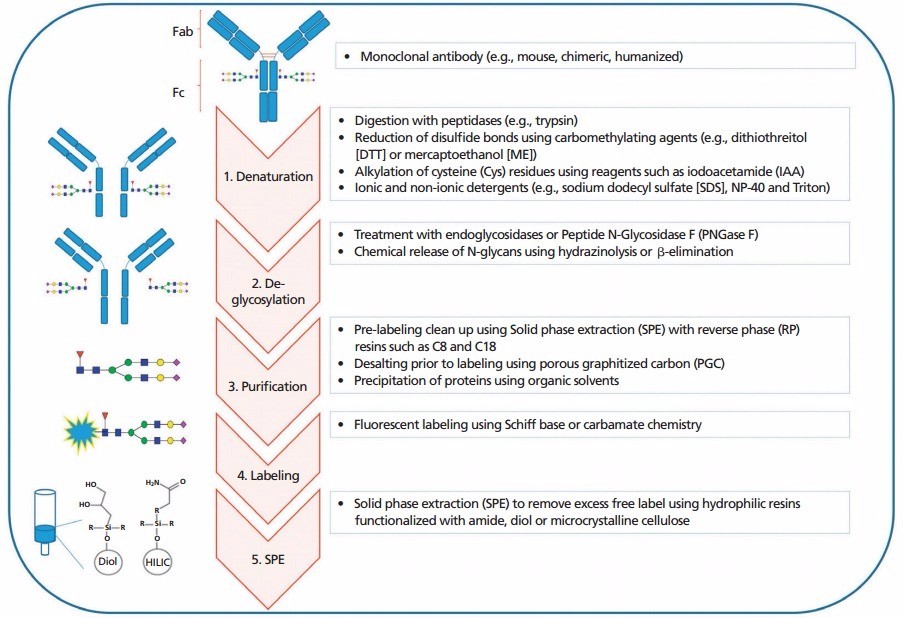
The General workflow for N-glycans profiling
Creative Proteomics can provide customized N-Glycan Profiling Service based on your needs.
- N-linked sugar analysis
- Analysis of peptides containing N-glycosylation sites
- Intact N-glycopeptide analysis