Everything needs a source of carbon to grow. For most microorganisms, CO2 is a feast, and in ecosystems, CO2 provides a source of carbon for everything to grow.
In almost all forms of life, the general pathway of energy metabolism is the tricarboxylic acid cycle (TCA cycle, also known as the Krebs cycle, discovered by Krebs, winner of the 1953 Nobel Prize in Physiology and Medicine). the TCA cycle converts the three major nutrients in food, sugar, lipids and protein, into energy and CO2. however, a recent report found that in some bacteria, the TCA cycle is reversed However, a recent report found that in some bacteria, the TCA cycle is reversed.
The research team of Ivan A. Berg from the University of Münster in Germany and the team of Wolfgang Eisenreich from the Technical University of Munich have collaborated to publish a study in Nature entitled High CO2 levels drive the TCA cycle backwards towards autotrophy. In this paper, the authors discovered a very curious cyclic process in bacteria - the REVERSE TCA cycle - that allows bacteria to thrive in an environment flooded with CO2 gas, providing new clues to the origin of the species.

Steffens and his colleagues studied the bacterium Hippea maritima. these microbes dislike oxygen, prefer high temperatures near 60°C, and derive energy from the reaction of hydrogen (H2) with sulfur to produce hydrogen sulfide (H2S). Like all life forms, they require a source of carbon to grow. And the resources available to them also depend on what is in the environment. If there is abundant protein around, H. maritima will use it as material to incorporate into the metabolic processes required for its growth.
When H. maritima grows at 40% CO2 concentration (1000 times higher than the atmospheric CO2 concentration), they undergo some kind of "chemical engineering" to exploit the reverse oxidative tricarboxylic acid cycle pathway. Steffens et al. used 13C carbon isotope labeling to track the CO2 eaten by Hippea maritima bacteria and recorded the accumulation of the intermediate molecule 13C to characterize the reverse TCA cycle in Hippea maritima bacteria.
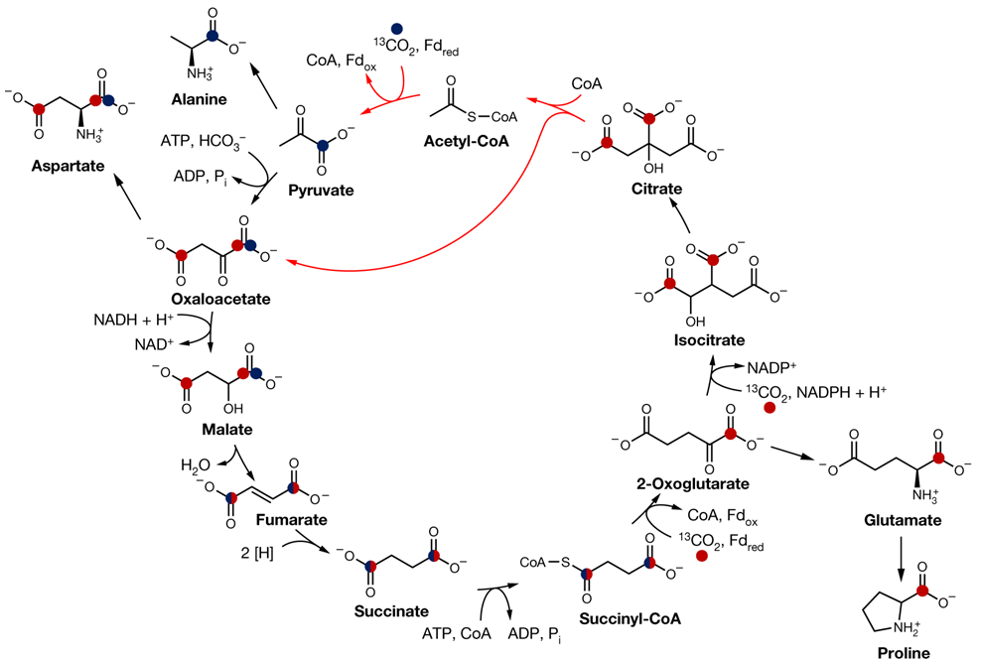
The roTCA cycle in H. maritima (Steffens et al., 2021).
This change in direction reverses the TCA cycle, converting CO2 into sugars, lipids and amino acids. Also in the same issue of Nature is a review Life in a carbon dioxide world by Martina Preiner and William F. Martin of the University of Düsseldorf, Germany, who suggest that in addition to CO2, hydrogen (H2) is also involved in the reverse TCA cycle in some bacteria, combining CO2 and H2 to form molecules such as amino acids, sugars and lipids.
This research provides new insights into microbial ecology, showing how life could have originated under conditions thought to be similar to those encountered by the first microbes on Earth: the organisms that gave rise to life were able to feed on CO2 to survive.
The tricarboxylic acid cycle plays an important role in multiple biosynthetic pathways. Abnormal TCA cycle function can lead to a variety of diseases. With comprehensive experience in isolation, characterization, identification and quantification, Creative Proteomics provides reliable, rapid and cost-effective TCA cycle metabolites analysis for your various scientific purposes.
Reference
Steffens, L., Pettinato, E., Steiner, T. M., Mall, A., König, S., Eisenreich, W., & Berg, I. A. (2021). High CO 2 levels drive the TCA cycle backwards towards autotrophy. Nature, 592(7856), 784-788.
